GENERAL
How to be a Good Guest
Where is Bat Cave?
The AREA
Area rock Layers
Topography
Geomorphology
Geological History
The Paleozoic
The Mesozoic
The Cenozoic
Water
The Hydrologic Cycle
Solution
Solution chemistry
Karst Landscapes
Erosional Features
Depositional Features
Environmental Issues
BAT CAVE
How was Bat Cave formed?
Surface Plan of the site
Map of the Cave
Life in and around Bat Cave
A Virtual Trip Through Bat Cave
TEST YOUR KNOWLEDGE
 KARST
PROCESSES
KARST
PROCESSES
Affecting some 15% of the land, karst processes and the resulting karst features, including caves*** and sinkholes, involve the dissolution of soluble rocks, such as gypsum, limestone, dolomite, rock salt, etc. . In carbonate rocks (limestones and dolomites mainly), the solution process is due to the chemical action of acidic water that dissolves away the rock. This creates a series of holes which increase in size over time. Extensive solution causes caves and caverns as well as sinkholes and other karst (=solution) features. Let's talk chemistry
How and why solution happens:
This part of the state is underlain by very thick limestone (calcium carbonate) and dolomite (calcium and magnesium carbonate) layers, that have been subjected to all sorts of processes since they were deposited. Some processes have been structural and created joints (cracks) and faults (cracks where the layers have moved) in the limestone. Many have been chemical in nature.
2. You have to have water
The water that does all this geological reshaping comes from rainfall
(or any precipitation for that matter..). The Gainesville area has plenty
of water. Every year on average some 54 inches of rain fall on the area.
But having enough water is not enough to bring about solution. By itself,
pure water would not especially affect limestones. That's because pure
water is neutral, neither acidic nor basic.
Where does the water in and around Bat Cave come from?
All water ultimately derives from the earth's oceans. Florida is no
exception. This constant exchange between oceans, land and life is often
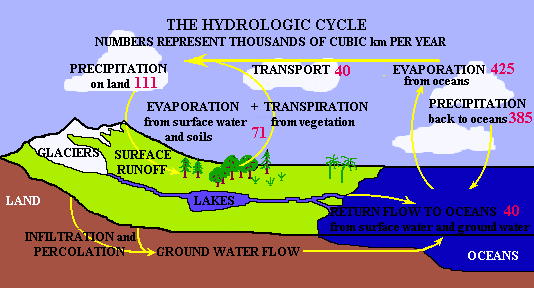
depicted as the hydrologic cycle, a great cycle that distributes water
and heat across the surface of the Earth. Earthwide, it is virtually a
closed system.
 A Simplified
Global
A Simplified
Global
Hydrologic Cycle
The hydrologic cycle in the Bat cave area is considerably simpler and, like any area, can be quantified in a water budget equation. The area receives water from precipitation and inflow from surrounding areas. It loses water through evaporation and transpiration and from outflow to other areas. Some of the water may be stored in lakes or ground water, and this stored amount may increase or decrease during wet or dry years. Some water is also used by us (consuptive use) and is not returned to the local system..
 Rainfall
Rainfall
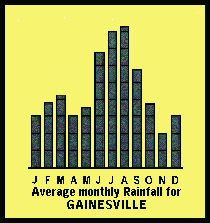
Annually, Alachua County receives some 54 inches of rainfall. June-September
are the wettest months. The least rainfall occurs in November. Most of
the winter rainfall is associated with cold fronts. In the summers months
rainfall is associated with convective thunderstorms and tropical storms
and hurricanes. Rainfall pH in the area is between 4.6 and 4.7.
Evapotranspiration
Evapotranspiration is around 34.5 inches/year, leaving a surplus of
nearly 20 inches. There are no lakes and streams (surface waters) in the
area because all the sediments and rocks in the area are highly porous
and permeable. Therefore, all the water that does not evapotranspire actually
infiltrates into the underlying Floridan Aquifer, which is the main aquifer
(ground water reservoir) and source of water in Florida.
Storage
This area is an area of high recharge to the Floridan Aquifer. The
main rock unit of this aquifer in the area is the Ocala Limestone, and
the underlying Avon Park Limestone. In this area, the Ocala Limestone is
very close to the surface. There are but a few feet (<20') of sediments
that overlie the limestone. As you can see as you descend the staircase,
in many places the limestone virtually outcrops at the surface.
What happens to the water in and around Bat Cave?
The general land elevation at the site is around 80-90 feet. While
the elevation of the potentiometric (water table) surface varies over time,
being lower in drought times and of course higher during wet periods, it
generally ranges between 35' and 40' MSL near the cave. In the aquifer,
waterflow is both diffuse, where the water filters between the grains
in the rock and conduit flow where water frows along enlarged openings
in the limestone such as lineaments and fractures. Water flow in
this part of the county is actually towards the northwest, generally heading
towards the Santa Fe River into which it discharges. No doubt as the area
becomes more developed, increasing mounts are discharged through wells.
Although some of this inflows again from irrigation, septic tanks etc,
much is also evaporated.
The great importance of the Floridan Aquifer as a water resource (it is the main source of potable water in the area) and the fact that this is an area of constant inflow (recharge) has led to increased concerns about the effect of human activities on the water supplies contained in the aquifer. For a long time, concerns mainly focused on industrial and urban activities. In contrast, little concern was expressed about the effects of human activities in rural areas, because of the low population densities. However, in the last three or four decades, we have come to realize that agriculture and other low density land uses can have as pronounced an effect on water quality as urban and industrialized areas. More on aquifer pollution
3. The water must be acidic
For water to be able to attack carbonates, it must be acidic. How does
water become acidic?
a) Atmospheric contamination4. This acidic water must be able to reach the carbonate rocks.
As water comes in contact with CO2 (carbon dioxide) in the atmosphere and in soils, water combines with carbon dioxide to make carbonic acid. Carbonic acid is a weak acid. Given the small amount of carbon dioxide in the atmosphere(~0.03%), we would expect rainwater to be mildly acidic (pH 5.5). But actually, the pH of raiwater is 4.8. In other words, rainwater is more acidic than expected. That's because CO2 in the atmosphere is not the only gas that affects how acidic precipitation becomes. Air pollutants such as sulfur and nitrogen oxides (produced by human and natural activity such as volcanos) also combine with water to form sulfuric and nitric acids and therefore add to the acidity of rainfall, i.e. lower its pH. As we continue to increase the amount of pollution we produce, we can expect that precipitation will become more acidic, significantly increasing solution rates over those we would expect to occur "naturally".
b) Soil contamination
As water continues its journey and infiltrates the soils, it further reacts with soil CO2 and becomes even more acidic. Some of this soil CO2 comes from bacteria and other micro-organisms that metabolize organic materials. Also, if the soil is rich in organic materials and therefore carbon, this carbon will combine with oxygen from the atmosphere to produce CO2. Because of bacterial activity and oxidation of carbon in soils, there can be 50 times more CO2 than in the soil than in the atmosphere. In addition, there are dissolved organic acids in the soil (e.g. tannic acid) which also contribute to acidity. The end result of all of these acid producing reactions is that the pH of waters in the soil or in the surficial aquifer may be as low as 3 and it is this acidic water that dissolves carbonates.
Areas where the water easily reaches the limestones either because the cover is thin, or permeable (such as sands) or where there are pathways that allow water to flow faster such as cracks in the rock layers (faults and joints) are all places where solution takes place at a much greater rate. In places where the limestone is better protected by greater thicknesses of less permeable materials such as silts and clays solution happens at a much slower rate.
Where does this happen most?
1. Greater amounts of solution occur in areas of recharge. Recharge areas are areas where surface waters (rainwater and occasionally stream water) infiltrate and replenish ground water as they inflow to deeper aquifers. As acidic water percolates downward in recharge areas it chemically attacks the limestone, most commonly near the top of the water table. It is much less active in discharge areas (areas where water is lost from ground water (such as in springs for instance) because these waters have already reacted with the carbonates and have lost their acidity and are now neutral or slightly basic. Keep in mind that lots of factors (such as changes in sea level) can change the areal extent of recharge areas.
2. Greater amounts of solution take place where the flow of acidic
waters is concentrated
 In
zones where water flow is greater such as along joints, fractures, faults
and along bedding planes (surfaces), carbonates are in contact with greater
quantities of acidic waters, and solution will concentrate its effects
along these areas of greater flow .
In
zones where water flow is greater such as along joints, fractures, faults
and along bedding planes (surfaces), carbonates are in contact with greater
quantities of acidic waters, and solution will concentrate its effects
along these areas of greater flow .
In this picture taken in the main room of Bat Cave, note how acidic
ground water has concentrated its activity along bedding surfaces in the
limestone creating what seems to be layers in the cave.
3. Greater amounts of solution happen where fresh and salt water
are mixing along the coasts. It turns out that if you mix
fresh and seawater (between 4-45 % sea water), even though either the fresh
and the salt water by themselves would not not lead to solution, the mix
of the two will actually dissolve some of the limestone. This is going
on today in coastal areas, and, because sea level has fallen and risen
repeatedly in the past, such mixing zones are much more prevalent
in the state than just in present coastal areas.
Average rates of solution vary tremendously, even locally, from virtually
none to rates as high as 1cm/100yrs. While this may not seem much, remember
the length of geologic time during which erosion operates.
On to Karst features
*** Caves can also form in lava flows (which of course is not an issue in Florida). But that method of cavity formation is totally different and not related to solutional karst processes.